What We Do
Research in the Strauss lab is conceptually motivated by questions in community and/or disease ecology. Most projects in the lab relates one or more of the following research themes:
- Relationships between biodiversity and disease
- Effects of predators on disease
- Thermal ecology of infectious disease
- Ecology and evolution of complex lifecycle parasites.
Most projects in the lab also focus on one of the following zooplankton study systems:
- A virulent fungal parasite that infects Daphnia in Midwestern lakes
- Microsporidian parasites that alternate between infecting cladocerans in the Southeast
- Guinea worm parasites that alternate between infecting copepods and humans in central Africa.
Research Themes
1) Relationships between biodiversity and disease
‘Dilution effects’ are patterns where increases in host diversity correspond with reductions in disease risk for a focal host species. This pattern can arise in zooplankton communities, when a) ‘diluter’ hosts consume parasites while rarely becoming infected, or b) ‘diluters’ compete with focal hosts, reduce their density, and inhibit density dependent disease transmission. However, ‘amplification effects’ can also occur, where levels of disease increase with added species diversity. Patterns of dilution vs amplification occur broadly in other communities of plants and animals for similar reasons. We are interested in building and testing models that predict how the likelihood of dilution or amplification varies with contexts including thermal conditions, predation regimes, host traits, evolutionary potential of hosts, and diversity of the parasite community. We approach these questions through a combination of field surveys in lakes and ponds, models parameterized with laboratory experiments, and mesocosm experiments that track changes in host community composition and infection patterns over multiple generations. Relevant publications: Strauss et al. 2015 Ecology Letters; Strauss et al. 2016 Ecological Monographs; Strauss et al. 2017 Proceedings B; Strauss et al. 2018 Functional Ecology; Strauss et al. 2024 Scientific Reports
left: Daniel and Kate prepare to sample at VIP pond, including assessment of the zooplankton and phytoplankton communities. top right: sampling out on the water. bottom right: Zooplankton differ substantially in body size, like these larger Daphnia and smaller Ceriodaphnia. Body size may be related to ability of zooplankton to inhibit harmful algal blooms.
2) Effects of predators on disease
How do predators affect disease dynamics in populations of their prey? The ‘healthy herds hypothesis’ suggests that predators can reduce disease in prey by a) selectively targeting and killing infected prey, b) reducing host density and density-dependent transmission, or c) altering stage structure of the host population in a way that favors disease transmission. On the other hand, predation can also increase disease risk if predators become infected themselves or if they serve as a paratenic host for the parasite. We are interested in the effects of fish predation on parasites that infect zooplankton, in both basic and applied contexts. Basic questions include asking how effects of predators on disease dynamics vary with temperature and host community composition, how their consumptive and non-consumptive effects compare, and whether seasonal changes in predation can explain the observed timing of disease outbreaks in the field. Our most applied question is how fish predation alters risk of transmission of Guinea worm parasites from copepods into people in Chad, Africa. We approach these questions through a combination of field surveys, parameterized models, and mesocosm experiments using mosquitofish as model predators. Relevant publications: Strauss et al. 2016 Ecological Monographs
far left: We maintain a colony of Gambusia mosquitofish which we use in experiments to study the effects of predation on disease. center left: T’Kai conducts a selectivity assay to measure whether fish preferentially attack healthy or infected prey. center & center right: Nadia conducts a mesocosm experiment to study effects of fish predation on copepod population dynamics. far right: Chaoborus larvae (top) are important invertebrate predators in both the Indiana lakes and Georgia ponds.
3) Thermal ecology of infectious disease
Warmer temperatures are predicted to increase the severity of infectious disease in many cases but not all. Thermal conditions can affect disease dynamics by altering traits of hosts, parasites, vectors, and any other taxa that influence disease spread. We are interested in a) how temperature affects disease dynamics at population and community scales, b) how thermal conditions interact with other variables such as nutrients or community context to affect disease, c) how seasonally cooling (in the fall) or warming environments (in the spring) relate to disease patterns in the field, d) and how fluctuations and variation in thermal conditions – rather than just mean temperature – affect disease. We approach these questions though a combination of field surveys, models parameterized by laboratory experiments conducted in incubators, and mesocosm experiments in large thermostatically-controlled water baths. Relevant publications: Shocket et al. 2018 Am Nat; Shocket et al. 2018 Ecology
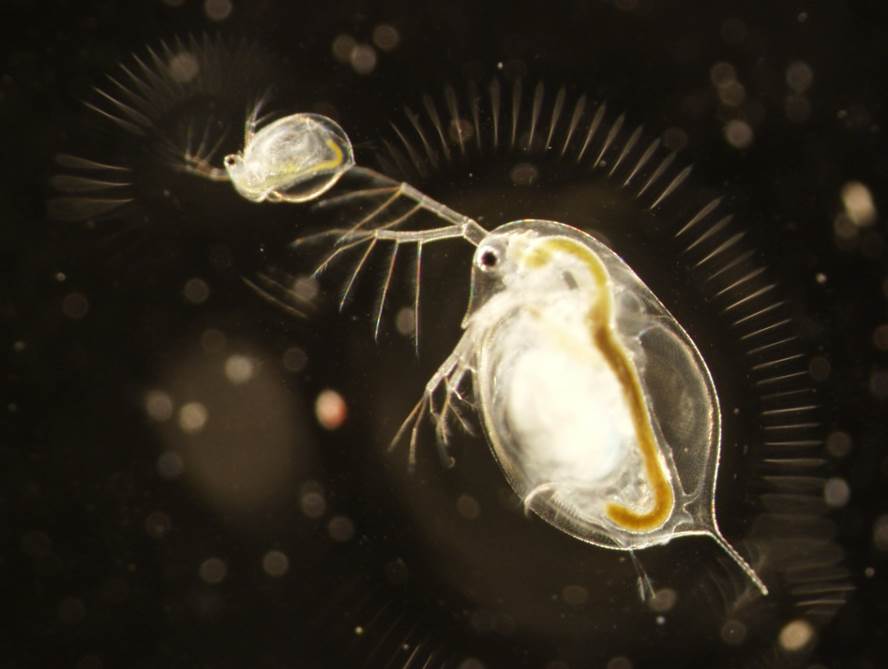
left: The host Daphnia dentifera infected by the fungus Metschnikowia bicuspidata. The first and third hosts are infected (notice the darker coloration due to fungal spores filling the host’s haemolymph). right: Mesocosm experiments that manipulate temperature (using giant water baths and water chillers/heaters) and community composition (e.g., presence of parasites, competitors, and/or predators).
4) Ecology and evolution of complex lifecycle parasites
Some parasites can complete their lifecycle by infecting a single host species, but others must infect a specific sequence of different species in order to reproduce. Several of the parasites that we study have complex lifecycles like this. Dracunculus medinensis (the causative agent of Guinea worm disease) alternates between infecting cyclopoid copepods and people, and the local microsporidian parasites likely alternate between cladocerans (with different species of parasite infecting Daphnia, Ceriodaphnia, Diaphanosoma, Simocephalus, and Bosmina) and chironomid midges. We are interested in ecological conditions that determine the timing and severity of outbreaks of complex lifecycle parasites, and evolutionary conditions that lead to these complex lifecycles and the breadth of hosts that parasites can infect at each stage. The microsporidian system is primarily field-based at the moment, although I am eager to develop it as an experimental system as well. Relevant publications: Strauss et al. 2023 Oecologia
left: Populations of Daphnia ambigua are heavily infected by the microsporidians Pseudoberwaldia daphniae and Conglomerata obtusa in early spring. Infected hosts appear darker in the center of their bodies. center: Daniel samples local ponds for communities of zooplankton and their parasites. right: Outbreaks of the microsporidians can reach up to 70%, like in this population of Daphnia laevis.
Study Systems

1) Metschnikowia bicuspidata is a virulent fungal parasite that infects Daphnia dentifera in upper Midwestern lakes. Hosts encounter the parasite while foraging, and infected hosts release parasite spores after death. Natural outbreaks occur in the fall, as lakes cool. The parasite’s lifecycle is completed entirely in D. dentifera, but other taxa influence disease dynamics. Outbreaks are bigger in lakes with more ‘sloppy’ Chaoborus insect predators, smaller in lakes with more intense fish predation, and smaller in lakes with greater zooplankton biodiversity and especially more Ceriodaphnia diluters. Neither this host nor this parasite live in Georgia, but we use this model system extensively in developing theory and testing it with mesocosm experiments.

2) Microsporidians are a fascinating and understudied group of eukaryotic parasites that are probably fungi. We recently discovered a group of several microsporidian parasites in ponds and reservoirs around Athens. Outbreaks occur in the spring as pond warm, infection prevalence can exceed 70%, and infections are nearly sterilizing for hosts. We regularly find six distinct parasite taxa, two of which closely match sequences of parasites previously only found in Europe. The others are new to science. Transmission is horizontal, and we are quite certain that these parasites have complex lifecycles with chironomid midges as definitive hosts. This is our primary field system, but experimental opportunities are limited for now.

3) Guinea worm parasites (Dracunculus medinensis) cause horrifying disease in people. Fortunately, thanks to massive global efforts led by the Carter Center, Guinea worm is now nearly completely eradicated. Only a handful of human cases arise each year, with cases restricted to Chad and other countries in central Africa. However, eradication efforts have stalled, as infections have been increasingly noticed in dogs in endemic areas. Our research mostly involves experiments to better understand the population dynamics of copepod hosts and their interactions with fish predators. These experiments support an ongoing field survey organized by our collaborators in Chad.
4) Fungal pathogens of grasses. One of these things is not like the others! We have a few projects that look at drivers and consequences of infectious disease in grasses. Along with local collaborators, we run a site as part of the DRAGNet (Disturbance and Resource Addition across global Grasslands) Network. We are interested in host biodiversity and eutrophication affect disease and herbivory in grasslands.